
The Neuromuscular Service at Leeds Children’s Hospital has received major funding to support pioneering research into treatments for neuromuscular conditions. The charities Duchenne UK and Leeds Hospital Charity have joined together to fund two roles at the service for five years. They are jointly donating over £300,000 to the service because of its track-record as a centre for excellence in paediatric neuromuscular care and leading-edge research. The service currently looks after nearly 500 children with neuromuscular conditions from across the Yorkshire region.
Urgent need for new muscular dystrophy treatments
Neuromuscular disorders are a diverse group of conditions that affect the muscles or the nerves that control them, leading to muscle weakness and other symptoms. While there has been progress in research into new treatments for some neuromuscular conditions, there is still a huge need for new and more effective treatments. This includes conditions such as Duchenne muscular dystrophy (DMD), the most common and severe type of childhood muscular dystrophy.
Researching new treatments
The neuromuscular service at Leeds Teaching Hospitals NHS has been at the forefront of research into new treatments. The team have delivered a number of ‘firsts’. They have been the first site to recruit to a number of DMD studies in the UK and recently delivered the first Phase 1 trial in the childrens’ research unit, looking at a new treatment for DMD. The team pride themselves on being able to deliver academic research, including studies into the impact of health inequalities on life with neuromuscular disease.
Giving Hope to families like Austin’s
11-year-old Austin was diagnosed with Duchenne Muscular Dystrophy (DMD) in November 2015, at just two years old. Austin’s mum Maxine initially thought he had a virus and took him to hospital, where he spent the next month having tests.
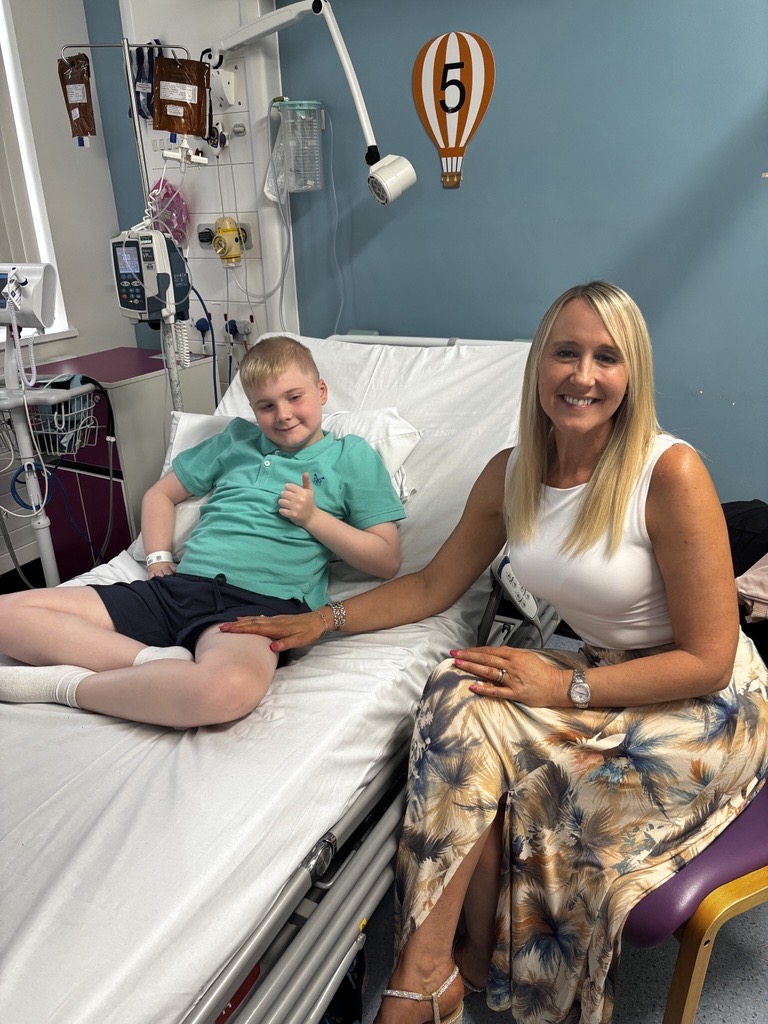
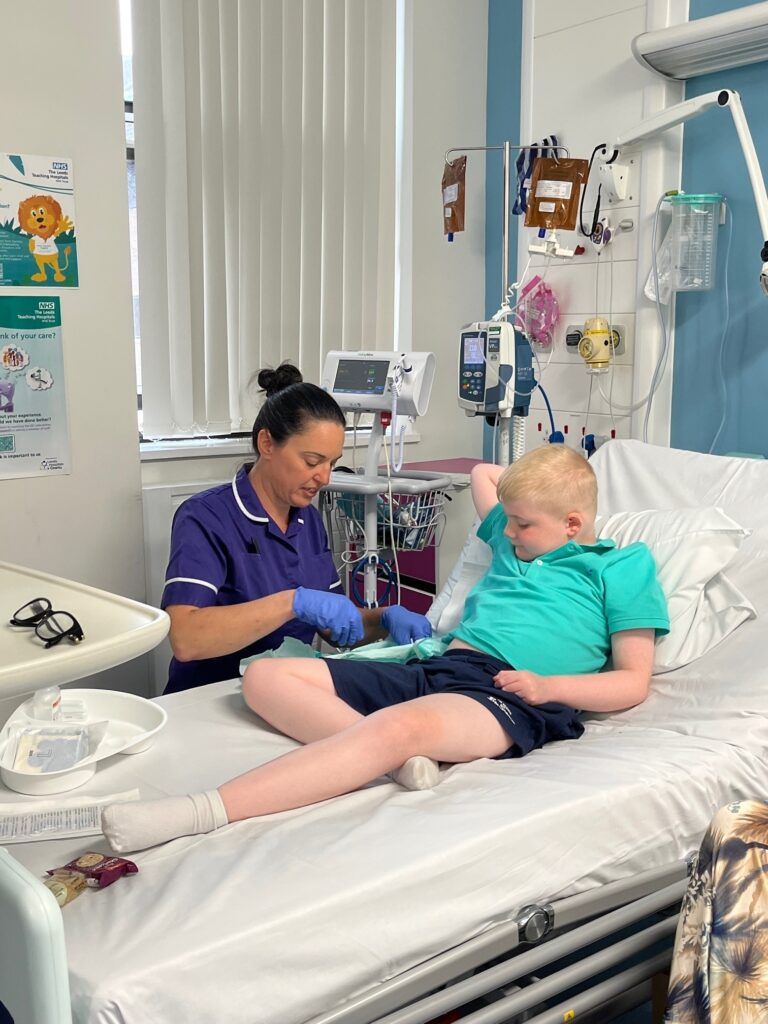
Three years ago, Austin began a trial at the Leeds Children’s Clinical Research Facility, with his family travelling over eight hours each week. Now, Austin has received over 130 infusions, he has a better understanding of his condition and knows he will lose the ability to walk, telling his mum he takes part in the trials because, ‘I want to help boys in the future to walk longer.’
For Austin’s family, the opportunity to participate in clinical trials has been a lifeline, offering hope to other families to find better treatments and eventually a cure. Austin’s current trial ends in September, but Maxine says if he chooses to continue to take part in research trials, they will continue to visit hospitals up and down the country.
‘From the moment Austin was diagnosed, our world changed. Nothing could have prepared me for the grief of knowing his life would be cut short. But through the darkness, clinical trials gave us hope. The support from the team in Leeds has been incredible; they’ve become like family. Research is vital, not just for medical breakthroughs, but for families like ours to feel less alone. Austin is paving the way, and we’re so proud of him.’
Austin’s Mum Maxine
Funding for roles
To sustain its current level of local and national engagement in clinical care and research activity, while maximising the opportunity to take on studies of Advance Therapy Investigational Medical Products (ATIMPS) including gene therapy trials, the service needed additional staff and more neuromuscular consultant time.
The funding from Duchenne UK and Leeds Hospital Charity will go towards funding the role of Consultant Paediatric Neurologist Dr Anne-Marie Childs. Dr Childs said the funding would be enable the service to plan for the future.
‘We’re incredibly grateful to both Leeds Hospitals Charity and Duchenne UK for their grant funding to support the children’s neuromuscular service. This investment will allow us to attract the best people to work in the Leeds neuromuscular team and create new opportunities for clinical trials that can support even more families. Neuromuscular conditions have a devastating impact, not just on the child affected but the whole family; funding like this gives hope that new treatments and new drugs can be developed.’
Dr Anne-Marie Childs
Recruiting for new Clinical Trials Coordinator
It will also fund a new Clinical Trials Coordinator, who will ensure that study protocols are delivered efficiently, releasing research nurse capacity and optimising the number of patients that can be seen and treated in the clinical trials unit. They will work with Dr Childs in seeking further sponsors for and investment in the work of the service.
Emily Reuben OBE, Co-founder and Chief Executive of Duchenne UK, said:
‘As the mother of a teenager who was diagnosed with Duchenne muscular dystrophy fifteen years ago, I remember the horror at discovering the lack of research into and treatments for his condition. That’s what led me to set up Duchenne UK and fund and support research since then.
While important progress has been made, there is a lot further to go. That’s why we are making this substantial donation to support Leeds Teaching Hospitals NHS Trust’s pioneering research.’
Esther Wakeman, Chief Executive of Leeds Hospitals Charity said:
‘Having a child diagnosed with a neuromuscular disorder is a life-changing event for any family. The neuromuscular service here in Leeds is already doing fantastic work in supporting patients and families from across the North of England; we’re thrilled to be making this investment so they can support even more families and continue the search for new treatments.’
If you would like to know more about or apply for the role of Clinical Trials Coordinator, you can visit NHS jobs here – Job Advert