
On Monday 7 July, Leeds Children’s Hospital welcomed visitors from The Natasha Allergy Research Foundation (NARF). Visitors from Natasha’s Foundation and their partner organisations came to see first-hand how The Natasha Clinical Trial is helping allergy patients in Leeds.
Children as young as two are taking part in the £2.7 million Natasha Clinical Trial across nine NHS hospitals including Leeds Children’s Hospital, part of Leeds Teaching Hospitals NHS Trust. The trial aims to demonstrate the effectiveness of commonly available foods to treat peanut or cow’s milk allergies.

The Natasha Clinical Trial, which began in 2023, is a randomised controlled trial – the gold standard in medical research. Before starting the treatment, the children and young people undergo food challenges over two hospital visits to confirm their food allergy diagnosis and make sure they are suitable to have the Oral Immunotherapy Treatment (OIT).
Once enrolled, each patient is given a very small amount of a food to which they are allergic at a dose that is safe for them, which is taken according to a standardised protocol under medical supervision. This may be given either in a hospital clinic or at home with medical support.
If they tolerate the food, they then take a daily ‘dose’ of this real food at home, and the amount is gradually increased by the medical team every few weeks while being closely monitored for any adverse effects.

It is hoped that the OIT treatment will mean that patients who had previously suffered anaphylaxis on exposure to products such as peanuts, will now be able to tolerate eating a small amount of the allergen without having a dangerous reaction.
The study aims to assess the effectiveness of OIT used to train the immune system of children and young people with food allergies to tolerate an allergen. The results and clinical experience gained from the trial will help shape future treatment options for food allergies in the NHS. Results from the trial are expected in 2027.
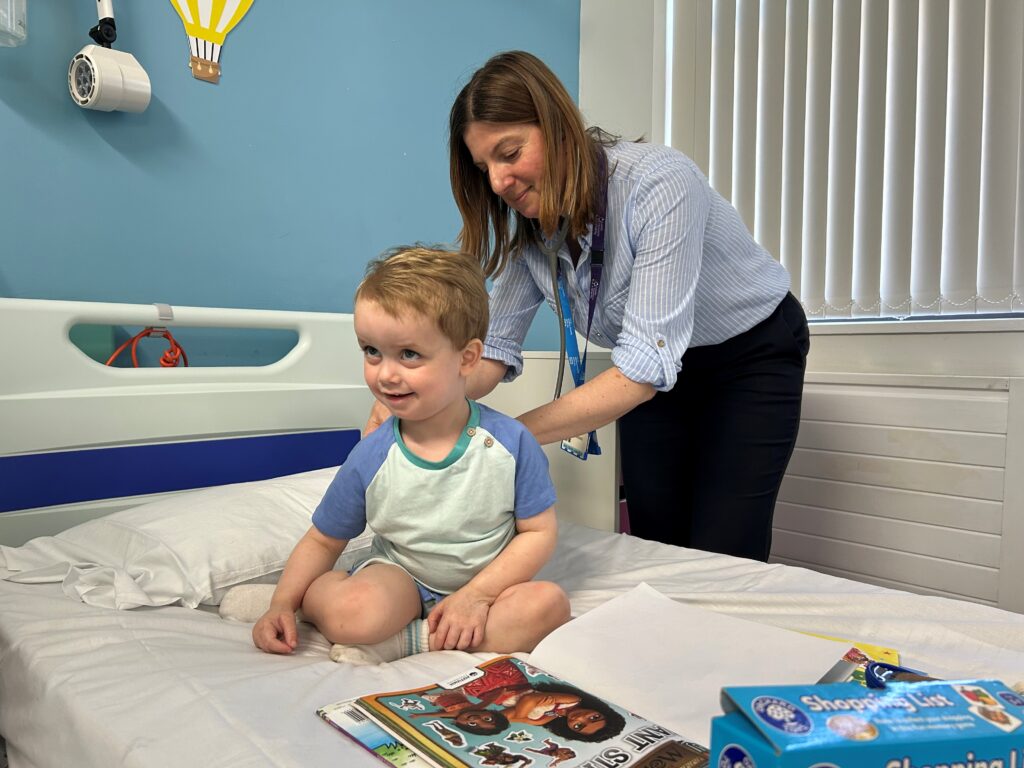
Visitors from Natasha’s Foundation were given a tour of Leeds Children’s Hospital facilities including the Children’s Allergy Day Unit where patients come for allergy treatments, and the NIHR Leeds Clinical Research Facility (CRF) Children’s Site where patients come to take part in the study.
The Leeds Children’s Hospital trial is being led by Dr Aida Semic-Jusufagic who said:
‘We’re delighted to welcome visitors from The Natasha Allergy Research Foundation and their supporters today and show them first-hand how their funding is already benefiting our patients here in Leeds. By taking part in this trial, we’re helping to shape the future of the treatment of allergy in the NHS’
The Natasha Clinical Trial is the first major study to be funded by The Natasha Allergy Research Foundation, set up by the parents of Natasha Ednan-Laperouse, who died aged 15 from a severe food allergic reaction.
Visitors spoke to families of patients taking part in The Natasha Clinical Trial in the CRF including parents of 2 year old Iyla who has several food allergies including dairy, egg, nuts, beans and peas. Parents Rachel and Mitesh spoke about how the Iyla’s dietary restrictions affect so many things in her life from child-care and holidays to day-day decisions about where to eat and who to spend time with. Iyla’s parents are delighted she has been given the opportunity to take part in The Natasha Trial.
Visitors from Natasha’s Foundation were joined by representatives from corporate partners Booths, CH&CO, Elior, Greene King and Samworth Brothers.
Rachel Hodson, Director of Corporate Partnerships and Fundraising at Natasha’s Foundation said:
‘It is great to see first-hand the amazing work going on at Leeds Children’s Hospital and meet some of the participants of The Natasha Clinical Trial and their families.
For our partners it was a fantastic opportunity to see how their donations are making a real difference to the lives of people with food allergies.’