- Home
- News
News
All the latest news and patient stories from Leeds Teaching Hospitals NHS Trust. Are you a journalist or from the media?
-

World Parkinson’s Day: Take Part in the PD Frontline study.
On World Parkinson's Day, we speak with one of our Lead Research Nurse, Prisca Mpofu, about Parkinson's research at Leeds Teaching Hospitals.
-
-

-
 This item has been tagged with the following terms:
This item has been tagged with the following terms: -

New Rob Burrow Centre for Motor Neurone Disease awaits planning approval
This item has been tagged with the following terms:Announcements, Blog, Community, Innovation, Our people, Research, Trust news
-

Children’s health experts gather for research partnership official launch
This item has been tagged with the following terms:Cancer, Children & Young People, Innovation, Research, Trust news
-
 This item has been tagged with the following terms:
This item has been tagged with the following terms:Blog, Children & Young People, Community, Digital, Innovation, Our people, Research, Sustainability, Trauma, Trust news
-

Double awards for Dr Rani Khatib, Consultant Pharmacist in Cardiology and Cardiovascular Research
This item has been tagged with the following terms: -
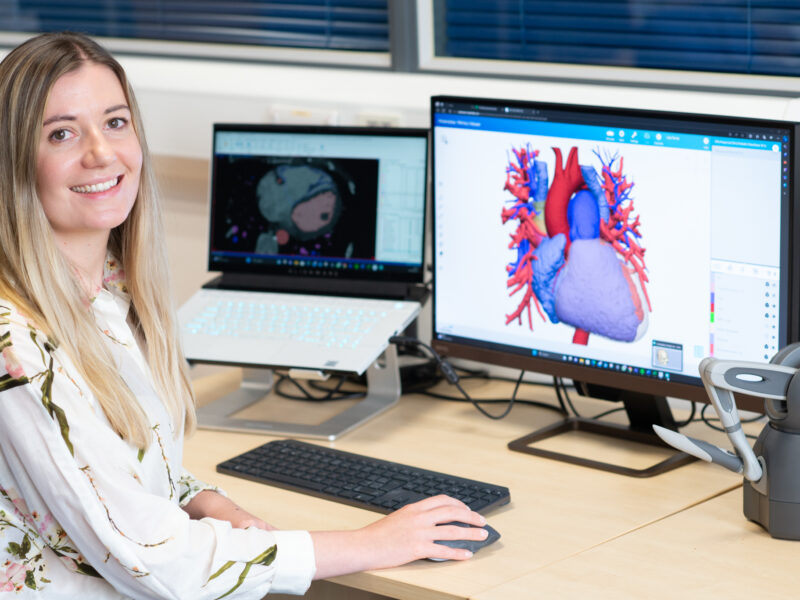
3D heart replicas help improve surgical outcomes for patients in Leeds
This item has been tagged with the following terms:Announcements, Children & Young People, Community, Heart, Our people, Research, Trust news
